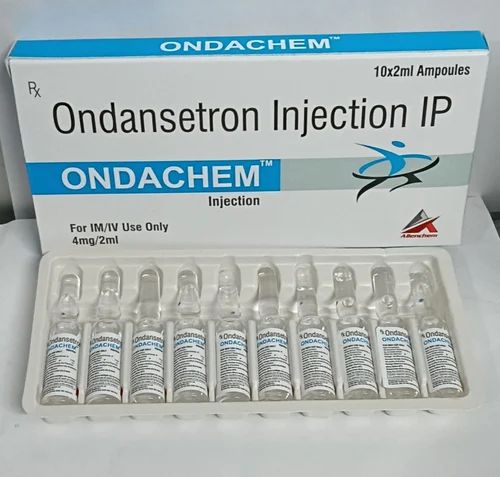
Deputy Minister of Health, Hansaka Wijemuni, confirmed that investigations are ongoing into a specific batch of the anaesthesia-related drug Ondansetron following reports of two patient deaths.
Speaking at a media briefing at the Kandy District Secretariat on December 16, Wijemuni explained that Ondansetron is routinely used to prevent side effects during anaesthesia. Millions of doses have been imported since April, with no adverse reactions reported until November.
Last month, five patients at Jayewardenepura Hospital experienced minor side effects. The National Medicines Regulatory Authority (NMRA) reviewed these cases and allowed continued use of the drug, while recommending further testing and careful monitoring.
Concerns escalated when a patient died on November 12 after receiving Ondansetron, prompting the NMRA to order hospitals nationwide to halt the use of the specific batch. A second death on November 14 involved a patient who had also received the drug. Authorities are now investigating whether the fatalities were caused by the medication or underlying medical conditions, and whether the batch contained harmful substances or had manufacturing defects.
The problematic batch has been withdrawn from all government and private hospitals, with instructions to source supplies from alternative importers, the Deputy Minister added.